DEVICE & COMBINATION PRODUCTS
At BlueRidge Life Sciences (BRLS), we serve as pharma’s device team for combination products—working seamlessly alongside your drug teams to accelerate development, streamline execution, and strengthen compliance.
Our experts bring expertise across quality management, regulatory preparedness, vendor selection and management, systems engineering, risk management, clinical and commercial development, human factors, and post-market readiness.
CORE AREAS OF EXPERTISE
-
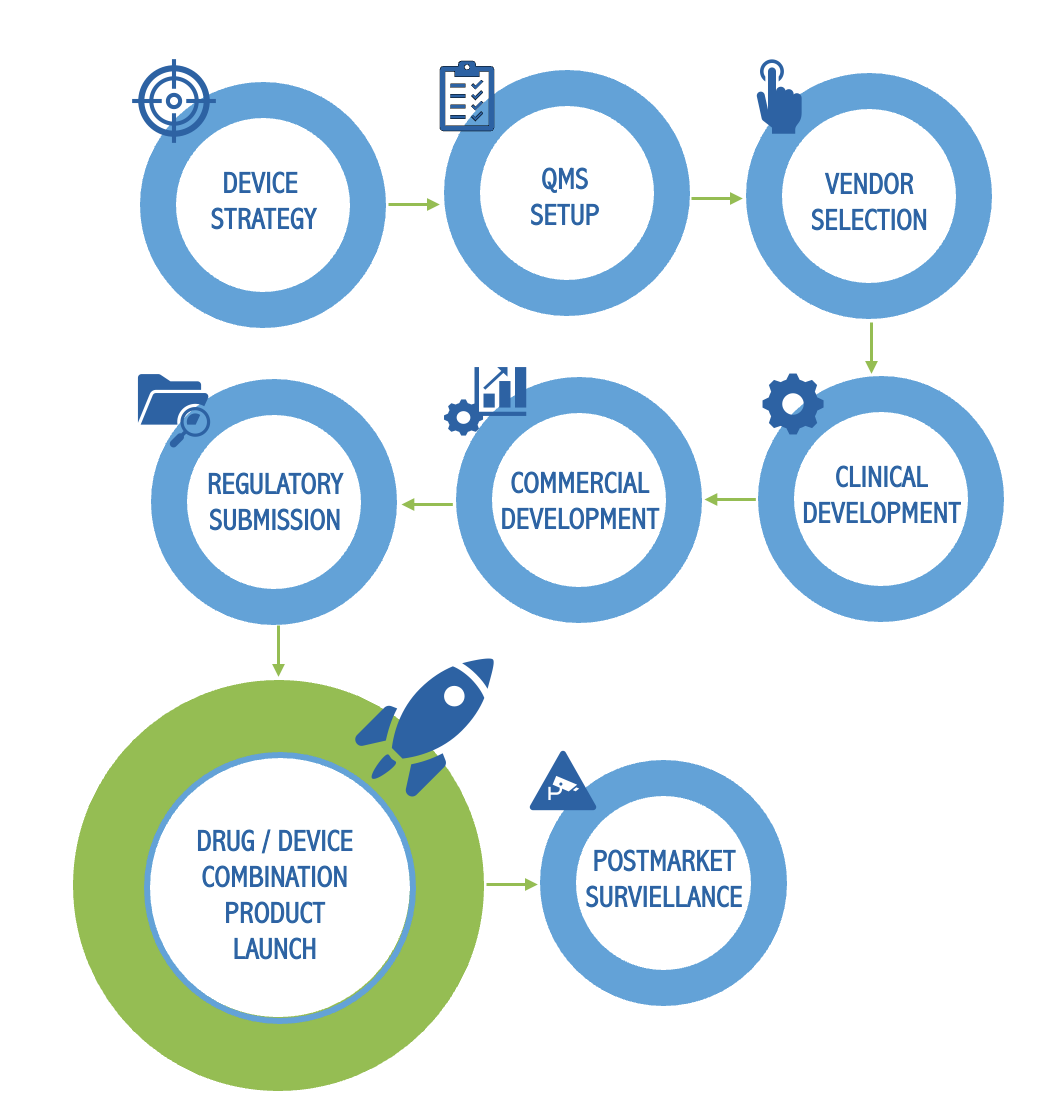
We provide managed services and integrated subject matter experts to support every phase of combination product development. From defining your product presentation to market through to post-market activities, our teams ensure strategy and execution align for a successful first-time approval and launch.
-
Building a strong and compliant combination product ecosystem requires alignment across leadership, systems, and culture. We help executive teams establish the organizational readiness needed to thrive in a rapidly evolving regulatory and competitive environment.
Services include:
Combination Product Strategy
Executive Coaching
Organizational Development
Education & Training
Corporate Readiness & Change
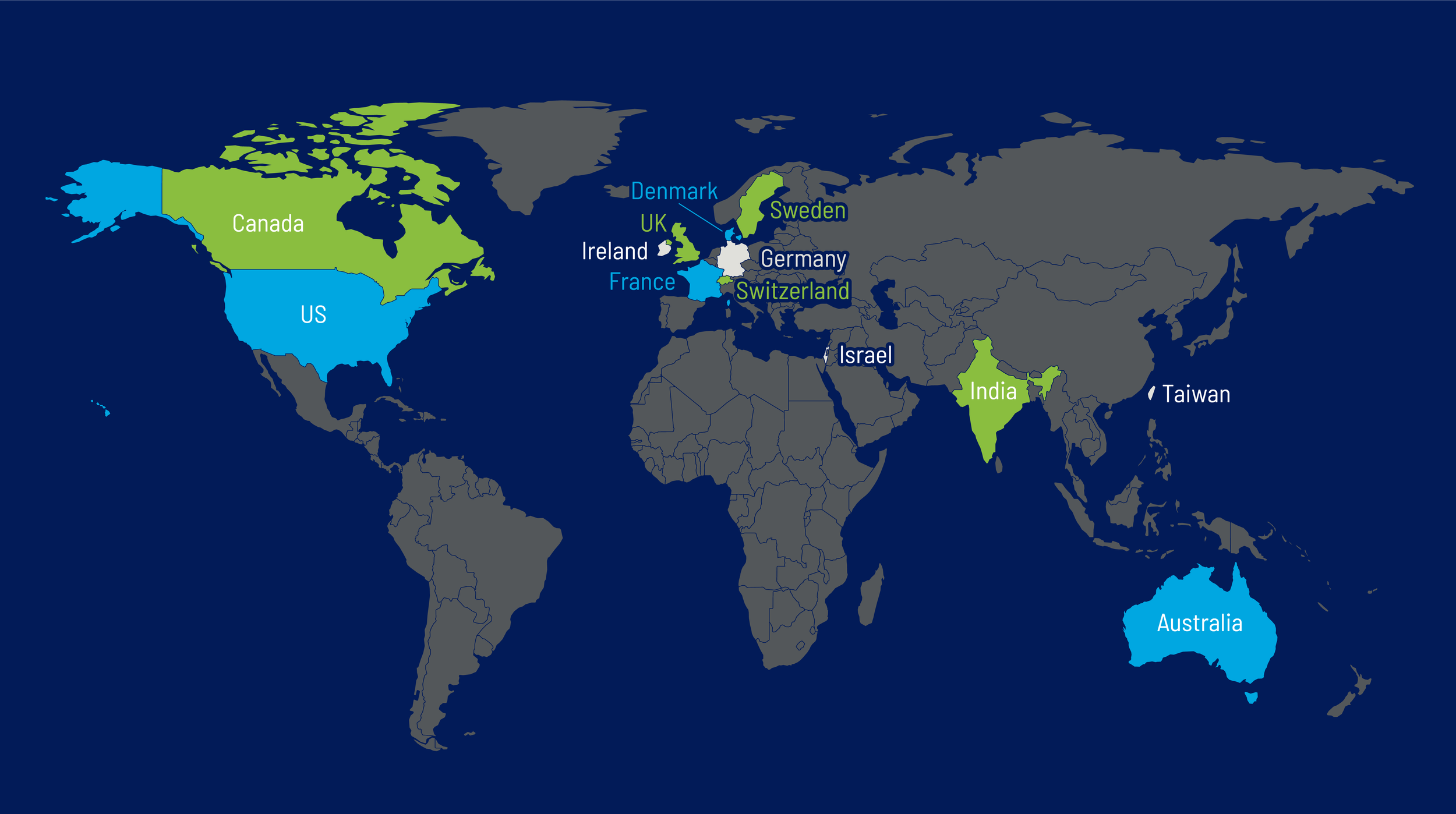
Our Global Clientele
Auto Injectors | Pre-Filled Syringes | On-Body Devices | Inhalers | Lyo-Kits | Attached Bio-Sensors | Vial Adaptors Adherence Support | Predictive Modeling Platforms
TAILORED, INTEGRATED, & SUSTAINABLE SOLUTIONS
Every combination product program requires stage-appropriate deliverables executed at the right time. At BRLS, we combine executive leadership coaching and program execution to design pathways tailored to your product’s lifecycle, risk tolerance, and corporate requirements. This complete-organization approach helps create a profitable and compliant combination product culture from the top down.
Our project teams are drawn from a broad pool of experts, carefully matched to your goals. With clear roles, responsibilities, and built-in oversight, our integrated teams adapt with agility as your needs evolve.
We work from the inside out—aligning device and drug processes, bridging gaps, and integrating compliant practices into your existing framework without disruption. By fostering cross-functional collaboration, applying customized solutions, and strengthening leadership, we help you build a sustainable combination product ecosystem that prospers alongside your core drug business.
Education and training are embedded into every engagement. We empower your internal teams to solve the next challenge, set up future improvements, and maintain long-term self-sufficiency.
Our Approach Includes:
Develop Tailored Roadmaps:
Clear plans for precise, stage-appropriate execution.
Support Corporate Readiness:
Strengthen processes and culture for lasting growth.
Deliver Subject Matter Expertise:
Program leadership and right-the-first-time execution at every stage.
Foster Collaboration:
Cross-functional alignment that accelerates progress.
Establish Compliant Foundations:
Build sustainable systems that support long-term success.
Equip Teams for Success:
Training and insights that empower long-term self-sufficiency.
CAPABILITIES THAT SET US APART
Proven methods that result in faster time to market, reduced risk, improved patient experience and adherence, and lower overall cost (higher profit)
700+ years of combined expert experience in device/drug combination product development
120+ combination product projects completed, creating a vast pool of global best practices
100+ SMEs from pharma, device OEMs, and regulatory bodies
Four former FDA / CDRH lead reviewers
Specialists experienced inside and with EU notified bodies
In-depth experience with SaMD and supporting software
Ability to assimilate at and support any phase of development
Training specialists to grow in-house knowledge
Consistent program management, technical leadership, and executive touchpoints across client programs

WHO WE SUPPORT
BlueRidge partners with pharma, biotech, startups, and device providers to deliver combination product expertise across routes of administration, therapeutic areas, and modalities.
-
Established Pharma and Biotech companies who have a dedicated in-house device team still often experience significant challenges due to resourcing issues and/or fast-evolving device delivery technology, regulatory landscapes, and processes needed to bring competitive combination products to market on-time and on-budget. Our experts are commonly brought in to execute:
Strategy + Corporate Readiness [Combination Product Strategy, Quality Systems Setup]
Combination Product Development [Device/Vendor Partnerships, Clinical Device Development, Regulatory Preparedness (Clinical Submission)]
Combination Product Commercialization [Commercial Device Development, Regulatory Preparedness (Commercial Submission), Commercial Launch, Post-market Activities]
Remediation + Readiness Improvement
-
Pharmaceutical and biotech startups without inhouse drug/biologic delivery device expertise rely on our experts to be their device team and combination product knowledgebase until internal scale up makes business sense. Our experts are commonly brought in to execute:
Combination Product Startup [Combination Product Strategy, Executive CP Coaching, Organizational Development]
Combination Product Readiness [Education + Training, Corporate Readiness + Change, Quality Systems Setup]
Combination Product Development [Device Selection, Regulatory Preparedness (Clinical Submission), Clinical Device Development]
Combination Product Commercialization [Commercial Device Development, Regulatory Preparedness (Commercial Submission), Commercial Launch, Post-market Activities]
-
We partner with drug delivery device manufacturers (OEMs and CMOs) and service providers (i.e., CROs and testing facilities) to elevate their value propositions and capabilities to support Pharma clients in the design, development, and adoption of their technologies. Our experts are commonly brought in to execute:
Turnkey Service Strategy [Assessment, Recommendations, Execution Support]
Quality Management Systems [Standard Operating Procedures, Integration of Pharma Device/Drug Quality Systems]
Corporate Readiness + Change
Education + Training
Audit Preparation [Product, Process, QMS, Regulatory]
Regulatory Support [Systems Alignment with Pharma, Regulatory Deliverables Preparation]
Post-market Surveillance [Compliance Process, Risk Management File, Triggers for Post-market Activities]
Ready to Advance Your Combination Product Development?
Contact us today to evaluate your device–drug strategy and discover how BlueRidge Life Sciences can help you streamline compliance, reduce risk, and accelerate product success.