Life Sciences Supply Chain Engineering
At BlueRidge Life Sciences (BRLS), Life Sciences Supply Chain Engineering refers to the structured design, assessment, and optimization of both physical and digital systems that govern the movement of regulated health products—from raw materials to patient or consumer use. We combine engineering principles, quality systems, and regulatory compliance to ensure product integrity and performance across every stage of the supply chain. We help to manage supply chains that are not only efficient, but also fully validated, risk-resilient, and audit-ready.
These products include:
Biologics
Cell and gene therapies
Medical devices
Diagnostics
Food ingredients
Other health-related products subject to regulatory oversight
What sets BlueRidge Life Sciences apart is our cross-functional approach, integrating:
Technical design (e.g., cold chain, labeling, device-packaging compatibility)
Regulatory and quality compliance (FDA, EMA, ISO, HACCP, etc.)
Risk management frameworks (including HAZOP and FMEA)
Digital infrastructure for serialization, traceability, and chain-of-identity
QMS maturity tailored to development phase and product complexity
We help clients move beyond traditional logistics and manufacturing mindsets — reframing the supply chain as a regulated, quality-controlled extension of the product itself, and as a strategic enabler of global market access, regulatory compliance, and patient safety
OUR CAPABILITY ASSESSMENT FRAMEWORK
To support our definition of Life Sciences Supply Chain Engineering, BlueRidge Life Sciences offers a structured capability assessment framework that evaluates supply chain readiness across six core domains. This methodology helps pinpoint strengths, identify risks, and prioritize improvements based on:
Your product’s risk profile
Its lifecycle stage
Your global market strategy
These six domains reflect the critical elements of regulatory-aligned supply chain design and execution in the life sciences industry.
-
End-to-end supply chain mapping
Product flow paths
Cold chain infrastructure
Node and facility design
-
HAZOP and FMEA workshops
ICH Q9 integration
Risk registries
Mitigation planning
-
Scalable Quality Management Systems (QMS)
Deviation and CAPA handling
Supplier quality oversight
Phase-appropriate controls
-
Serialization, chain of identity, and chain of custody
Electronic Batch Manufacturing Records (eBMR)
Real-time tracking
System integration
-
Shipper validation
Device interface testing
Regulatory harmonization
Label control systems
-
Depot and partner audits
Clinical and commercial launch readiness
Global distribution performance

MATURITY LEVELS
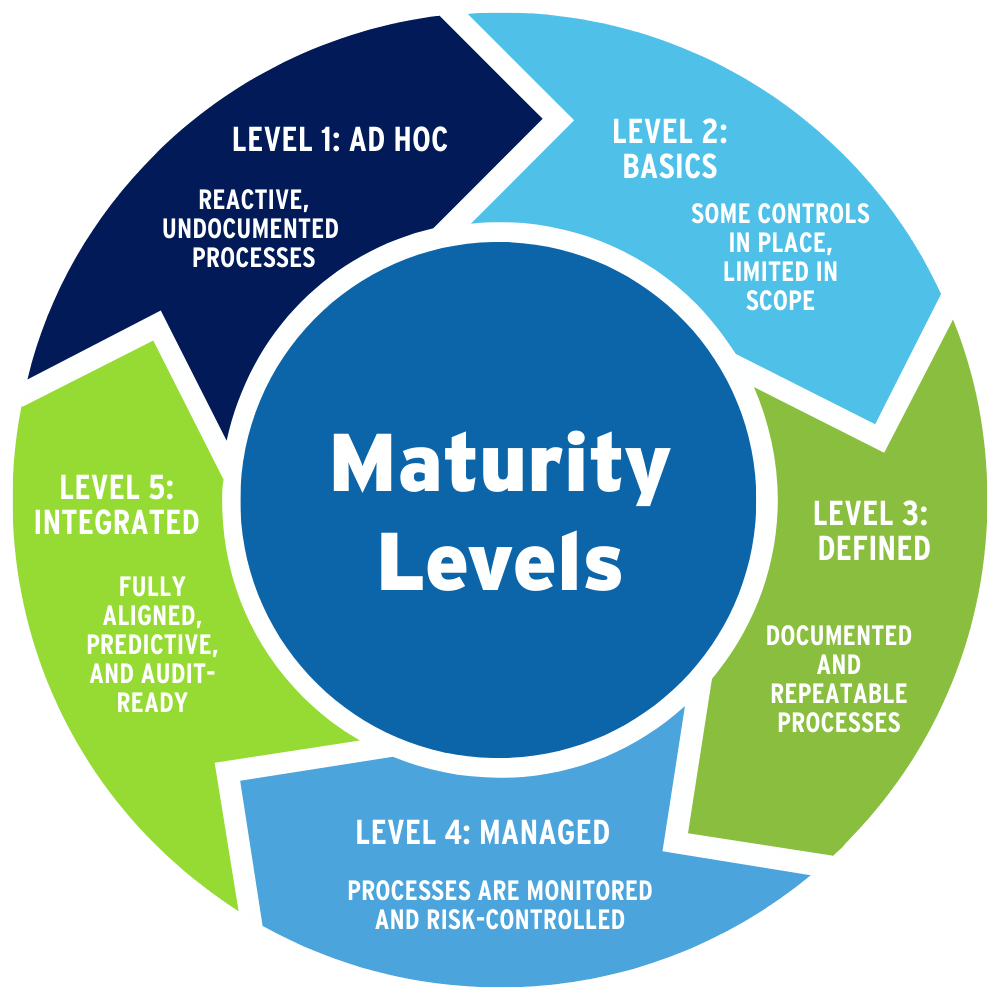
Each of the six capability domains is evaluated using a standardized 5-level maturity model. This helps organizations understand their current state and identify where targeted improvements will deliver the greatest impact. This scoring approach supports benchmarking, prioritization, and alignment with regulatory expectations throughout the life sciences supply chain.
Maturity Levels:
Level 1 – Ad Hoc: Reactive, undocumented processes
Level 2 – Basic: Some controls in place, limited in scope
Level 3 – Defined: Documented and repeatable processes
Level 4 – Managed: Processes are monitored and risk-controlled
Level 5 – Integrated: Fully aligned, predictive, and audit-ready

HOW WE DELIVER IT
We offer our supply chain capability assessment in flexible formats to meet a range of timelines and project needs. Each delivery model is designed to uncover operational risks, validate quality systems, and align your life sciences supply chain with product and regulatory requirements.
Rapid Workshops:
2-3 Days
Collaborative mapping sessions
Guided walkthroughs of risk and system readiness
Full Diagnostics:
2-6 Weeks
In-depth stakeholder interviews
Evidence and documentation review
Scoring and detailed reporting
Integrated Readiness Reviews:
3+ Months
Embedded support for:
Commercialization planning
M&A due diligence
IND/BLA preparation
WHAT YOU RECEIVE FROM A SUPPLY CHAIN CAPABILITY ASSESSMENT
Our life sciences supply chain assessment delivers actionable outputs that support regulatory strategy, operational improvement, and quality assurance. You’ll receive:
Capability Maturity Scorecard: A visual heatmap across all six domains
Gap and Risk Report: Includes detailed risk assessment findings and QMS status
Prioritized Roadmap: Clear next steps aligned with your development stage and regulatory pathway
Optional: Regulatory Submission Support: Support for validation summary language and CMC linkages to strengthen filing

WHY SUPPLY CHAIN READINESS MATTER IN LIFE SCIENCES
In the life sciences industry, supply chains are not just logistical functions—they’re strategic assets that enable regulatory approvals, protect product stability and integrity, and ensure patient safety.
Whether you're scaling a monoclonal antibody (mAb) therapy, launching a diagnostic kit, or commercializing a novel food-grade ingredient, our assessment framework gives you the tools to move forward with confidence—armed with insights, compliance readiness, and a validated plan for success.
Ready to Strengthen Your Supply Chain Strategy?
Contact us today to assess your supply chain readiness and learn how BlueRidge Life Sciences can help you enhance compliance, reduce risk, and accelerate access to global markets.