Nonclinical Safety
CORE AREAS OF EXPERTISE
BlueRidge Life Sciences Nonclinical Safety helps pharmaceutical and biotech clients design, manage, and optimize nonclinical programs with the scientific rigor and regulatory foresight needed to move safely and efficiently toward clinical trials. Our team supports more than 30 therapeutic areas and modalities, including traditional small molecules, biologics, cell and gene therapies, and rare disease treatments.
-
We guide clients through early development with precision, foresight, and confidence:
Develop integrated toxicology strategies tailored to indication, route, and patient population
Identify appropriate study models and address challenges such as species selection — including programs with no relevant species
Facilitate regulatory meetings and guide toxicology response strategies across FDA, EMA, Health Canada, Australia, New Zealand, and MHRA
Provide strategic regulatory guidance and document review throughout development
-
We oversee critical GLP and non-GLP studies and translate results into actionable insights, seamlessly integrating them into early-phase clinical development strategies to drive informed, accelerated decision-making:
Manage a wide variety of studies ranging from in vitro toxicology to general toxicology to reproductive and developmental to carcinogenicity and more
Guide clinical dose selection, escalation strategies, and clinical monitoring plans
Ensure alignment between nonclinical strategy and clinical program goals
Represent toxicology in cross-functional development discussions and at regulatory meetings
-
We help clients prepare regulatory filings that comply with global standards, ensuring seamless approval pathways and accelerated time to market:
Nonclinical sections for INDs, CTAs, NDAs, BLAs, and CTDs
Prepare and review nonclinical expert reports and supporting documentation
Develop tabulated and narrative summaries for pharmacokinetics and toxicology
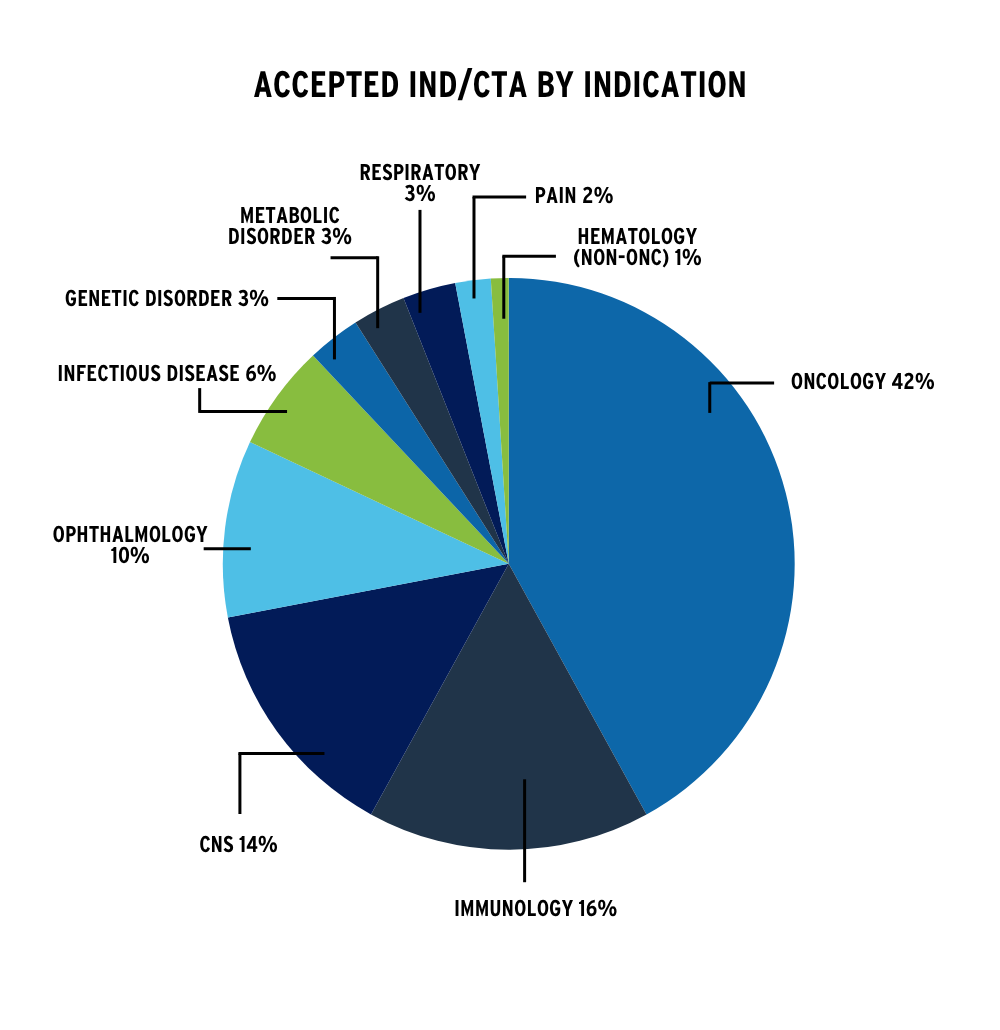
Contribute to 70+ INDs/CTAs submitted since 2012

STRATEGIC SOLUTIONS IN NONCLINICAL DEVELOPMENT
Flexible Approaches for Rare Disease Programs
For rare and serious conditions, nonclinical safety programs often require customized approaches. BlueRidge helps clients:
Align with regulatory guidance that allows shortened or reduced toxicology packages
Establish First-in-Human doses
Navigate pediatric requirements, including justification for deferring or omitting juvenile toxicity studies when appropriate
Address global differences in requirements for rare disease indications
Support programs with no relevant species through alternative model strategies
Data-Driven Risk Assessment and Modeling
We drive smarter, earlier decision-making through risk-informed assessments and advanced predictive tools. Our team helps clients:
Prepare carcinogenicity and reproductive toxicity assessments
Develop monographs for excipients and impurities
Assess metabolite safety to meet regulatory thresholds
Use (Q)SAR models like Derek Nexus, Leadscope, and Meteor
Justify waivers and streamline regulatory submissions with data-backed evidence
SPECIALIZED CAPABILITIES THAT SET US APART
Target Liability and Risk Assessments
Metabolite Safety Qualification and Assessment
Impurity and Excipient Liability Assessments
Expertise in Programs with No Relevant Nonclinical Species
Strategic Regulatory Guidance and Planning
Due Diligence Support (In- and Out-Licensing)
Preparation of Nonclinical Expert Reports
Regulatory Document Preparation and Review
Poject Team and Regulatory Meeting Attendance
Support for Specialized Routes of Administration

THERAPEUTIC AREAS & MODALITIES WE SUPPORT
BlueRidge supports a wide range of product types and therapeutic areas, from small molecules to gene therapies.
-
Intravenous, subcutaneous, intrathecal, inhalation, dermal, oral and more
-
Traditional drugs, botanicals, peptides, oligonucleotides
-
Antibodies, ADCs, biosimilars, fusion proteins, bispecifics, vaccines, immunotherapies
-
Cell and gene therapies, novel constructs
-
Oncology, neurology, ophthalmology, autoimmune disease, metabolic disorders, cardiovascular, rare diseases, and more
Our experience spans a diverse portfolio of therapeutic modalities and indications, reflecting the depth and versatility of our nonclinical safety expertise. The IND acceptances shown here represent successful regulatory outcomes across a wide range of molecule types and therapeutic areas.
Ready to Build a Stronger Nonclinical Foundation?
Contact us today to discuss your development program and learn how BlueRidge can help you reduce risk, streamline timelines, and move forward with confidence.