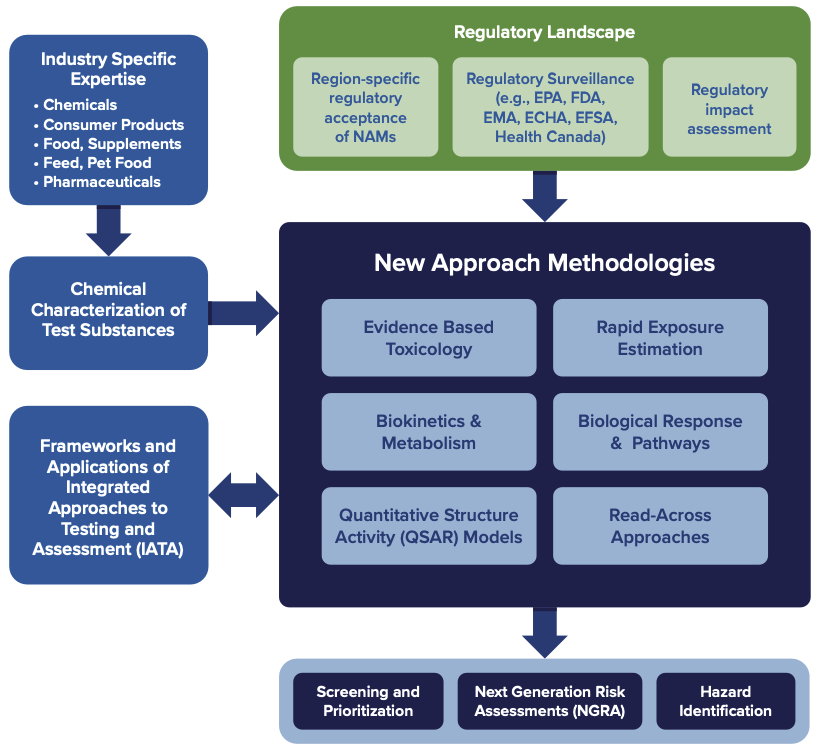
New Approach Methodologies (NAMs)
At BlueRidge Life Sciences (BRLS), we support organizations as they navigate the evolving use of New Approach Methodologies (NAMs) to make confident, regulatory-aligned safety decisions.
NAM tools are increasingly accessible, but their value depends on how well they are integrated, interpreted, and positioned for regulatory acceptance. What distinguishes BRLS’ NAMs services is the integration of scientific rigor, exposure context, and regulatory insight to support confident, informed safety assessments that reduce or replace animal testing while maintaining scientific and regulatory credibility.
Grounded in extensive experience supporting safety decisions across regulated industries, our team brings practical, domain-specific expertise to the application of NAMs. This experience informs early screening and prioritization, product stewardship and chemical safety assessments, and global regulatory submissions across the chemical, cosmetics, pharmaceutical, food, and consumer product sectors. This enables faster, more human-relevant decision-making without compromising compliance.
CORE AREAS OF NAMS EXPERTISE
BRLS applies NAMs through a structured, evidence-based approach that integrates multiple data streams to support robust safety evaluations.
-
We apply QSAR models, read-across approaches, machine learning and deep learning methods, physiologically based toxicokinetic (PBTK) modeling, and AI-based prediction tools to support hazard identification and risk assessment.
-
High-throughput toxicokinetic models, reverse dosimetry, and exposure estimation methods are used to place NAM outputs into realistic human exposure context.
-
Adverse Outcome Pathway (AOP) frameworks and read-across strategies are used to support biologically relevant interpretation of NAM data and strengthen confidence in hazard identification and risk assessment.
-
We integrate biological activity data with exposure and mechanistic context to support transparent, weight-of-evidence toxicological evaluations, incorporating systematic review methods, qualitative and quantitative study ranking, risk-of-bias assessment, and uncertainty characterization.
-
Our approach includes conventional cell-based assays, high-throughput screening methods (such as ToxCast), and high-content screening technologies; including human-relevant in vitro models, omics approaches, and cell painting assays.
We apply NAMs in accordance with the current state of the science, following established regulatory guidance and peer-reviewed methodologies. This includes alignment with frameworks and guidance from organizations such as ICCVAM and the OECD, including guidance for QSAR application, grouping, and read-across.
We also support region-specific regulatory acceptance of NAMs through ongoing regulatory surveillance and regulatory impact assessment across key global markets, including:
North America – U.S. Environmental Protection Agency (EPA), U.S. Food and Drug Administration (FDA), and Health Canada
United Kingdom and European Union – Medicines and Healthcare products Regulatory Agency (MHRA), European Medicines Agency (EMA), European Chemicals Agency (ECHA), and European Food Safety Authority (EFSA)
Asia – Japanese regulatory authorities, including agencies involved in chemical and pharmaceutical safety oversight
SCIENTIFIC AND REGULATORY ALIGNMENT
AN INTEGRATED FRAMEWORK FOR APPLYING NAMS

WHERE NAMS APPLY ACROSS THE PRODUCT LIFECYCLE
NAMs support safety decision-making across a range of product types and development stages, including industrial chemicals, food and flavor ingredients, consumer products and cosmetics, and pharmaceuticals. By integrating NAMs across development stages, BRLS supports safety decisions from early innovation through regulatory submission and post-market evaluation.
-
Early discovery, screening, and prioritization
Formulation and product optimization
Read-across and next-generation risk assessments (NGRA)
Regulatory submissions and decision-making
Post-market safety evaluations
Supported substance types include small molecules, excipients, impurities and degradants, and new chemical introductions—from early-phase R&D through late-stage regulatory and stewardship programs. By integrating NAMs across development stages, BRLS supports safety decisions from early innovation through regulatory submission and post-market evaluation.
HOW WE DELIVER NAMS-DRIVEN INSIGHTS
We provide human-relevant, decision-ready safety insights derived from the integration and interpretation of in vitro, in silico, and exposure-based data. Outputs are tailored to client needs and aligned with regulatory expectations.
Deliverables may include:
Regulatory-aligned or custom safety assessment reports
Frameworks to evaluate and build confidence in NAM outcomes
Dashboards that support exploration of multiple NAM data streams
Exposure and toxicokinetic modeling outputs
In silico model predictions
Read-across assessments
In vitro data summaries
Engagement models can include technical consultations, facilitated workshops, embedded project support, or advisory packages.

CLIENTS WE SERVE
Our NAMs services support organizations that require efficient, human-relevant safety evaluations across regulated product lifecycles.
-
Chemical manufacturers
Supporting substance evaluation, screening, prioritization, read-across, and regulatory safety assessments across new and existing chemicals.Consumer product companies
Informing product stewardship and ingredient safety decisions while reducing reliance on animal testing.Food and supplement developers
Providing human-relevant safety insights to support ingredient assessments, formulation decisions, and regulatory submissions.Feed and pet food manufacturers
Supporting safety evaluations for ingredients and formulations across regional regulatory frameworks.Government research agencies
Supporting endocrine disruption screening, in vitro assay development and application, AOP development, systematic review, and evidence-based safety evaluations.Pharmaceutical companies
Applying NAMs to support early screening, mechanistic understanding, and safety decision-making across development programs.
-
Toxicologists and risk assessors
Interpreting NAM data within a defensible, weight-of-evidence framework.Regulatory scientists and regulatory affairs professionals
Supporting region-specific regulatory acceptance and regulatory-ready documentation.Product stewardship teams
Informing chemical safety assessments and ongoing compliance obligations.Product discovery teams
Supporting early screening, prioritization, and decision-making to guide compound and ingredient selection.R&D leaders
Enabling faster, data-driven decisions early in the innovation cycle.
Ready to Apply NAMs with Confidence?
Contact us today to learn how BlueRidge Life Sciences can help you integrate and interpret NAM data to support human-relevant, regulatory-aligned safety decisions across the product lifecycle.